Mohammad Mosharraf Hossain, Isuru M. Jayalath, Renuka Baral, and C. Scott Hartley*
ChemSystemsChem 2022, 4, e202200016
[Published version | DOE-PAGES | Preprint | Raw data]
Abstract
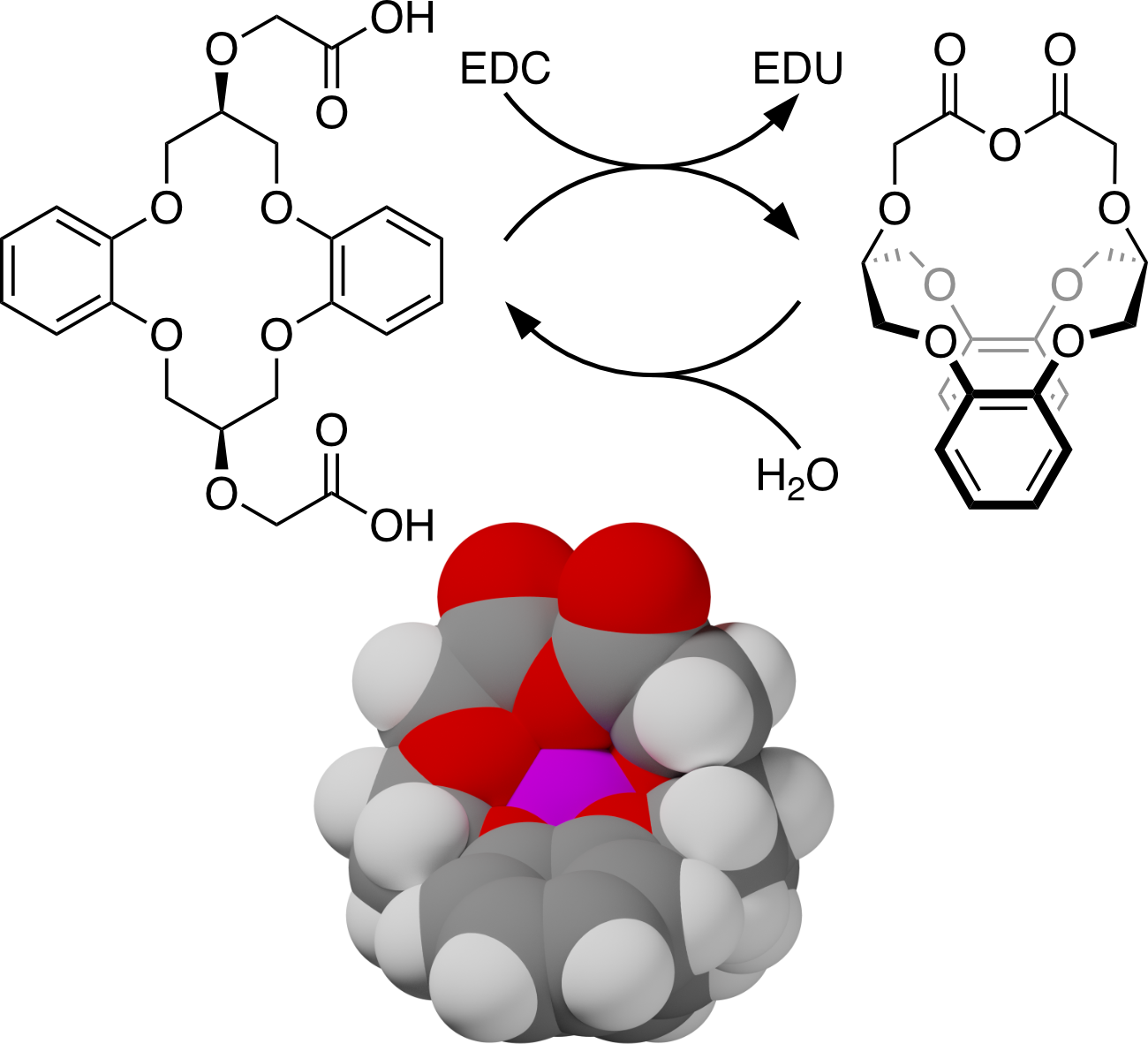
The use of “fuel” compounds to drive chemical systems out of equilibrium is currently of interest because of the potential for temporally controlled, responsive behavior. We have recently shown that transiently formed crown ethers exhibit counterintuitive templation effects when generated in the presence of alkali metal cations: “matched” cations, such as K+ with an 18-crown-6 analogue, suppress the formation of the macrocycles (negative templation). In this work, we describe two macrocyclic diacids that, on treatment with carbodiimides, give transient macrobicyclic cages analogous to polyether cages. Negative templation effects are observed for the smaller cage when generated in the presence of K+ and Na+, but there is a weak, but reproducible, positive templation effect in the presence of Li+. The larger cage behaves similarly in the presence of Li+, K+, Rb+, and Cs+, but differently with Na+, which appears to bind to both the cage and the initial macrocycle.
