Isuru M. Jayalath, Hehe Wang, Georgia Mantel, Lasith S. Kariyawasam, and C. Scott Hartley*
Org. Lett. 2020, 22, 7567–7571
[Published version | DOE-PAGES | Preprint | Raw data]
Abstract
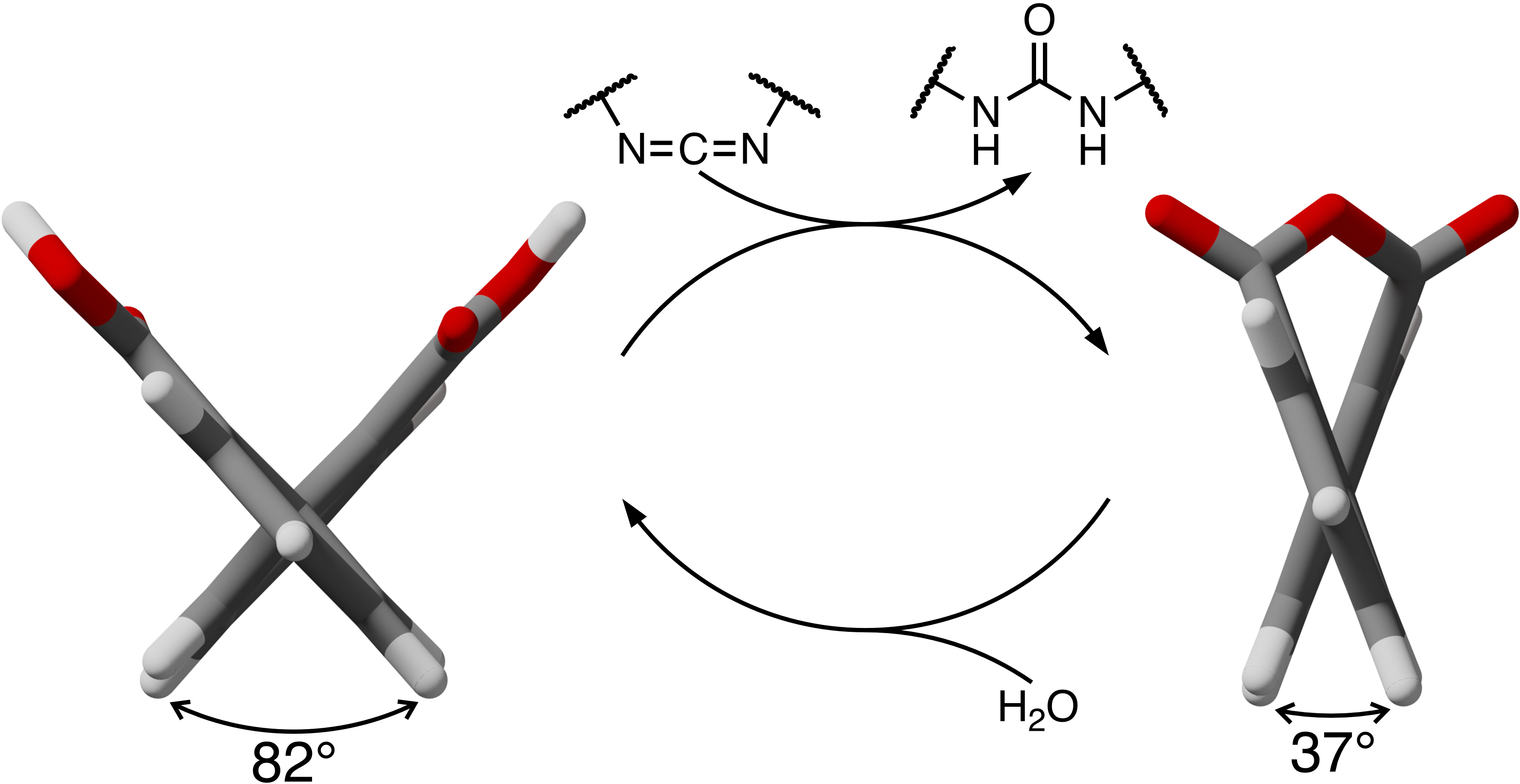
Transient changes in molecular geometry are key to the function of many important biochemical systems. Here, we show that diphenic acids undergo out-of-equilibrium changes in dihedral angle when reacted with a carbodiimide chemical fuel. Treatment of appropriately functionalized diphenic acids with EDC (N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride) yields the corresponding diphenic anhydrides, reducing the torsional angle about the biaryl bond by approximately 45°, regardless of substitution. In the absence of steric resistance, the reaction is well-described by a simple mechanism; the resulting kinetic parameters can be used to derive important properties of the system, such as yields and lifetimes. The reaction tolerates steric hindrance ortho to the biaryl bond, although the competing formation of (transient) byproducts complicates quantitative analysis.
