William S. Salvia, Georgia Mantel, Nirob K. Saha, Chamoni W. H. Rajawasam, Dominik Konkolewicz,* and C. Scott Hartley*
Chem. Commun. 2024, 60, 12876–12879
[Published version | DOE-PAGES | Preprint | Raw data]
Abstract
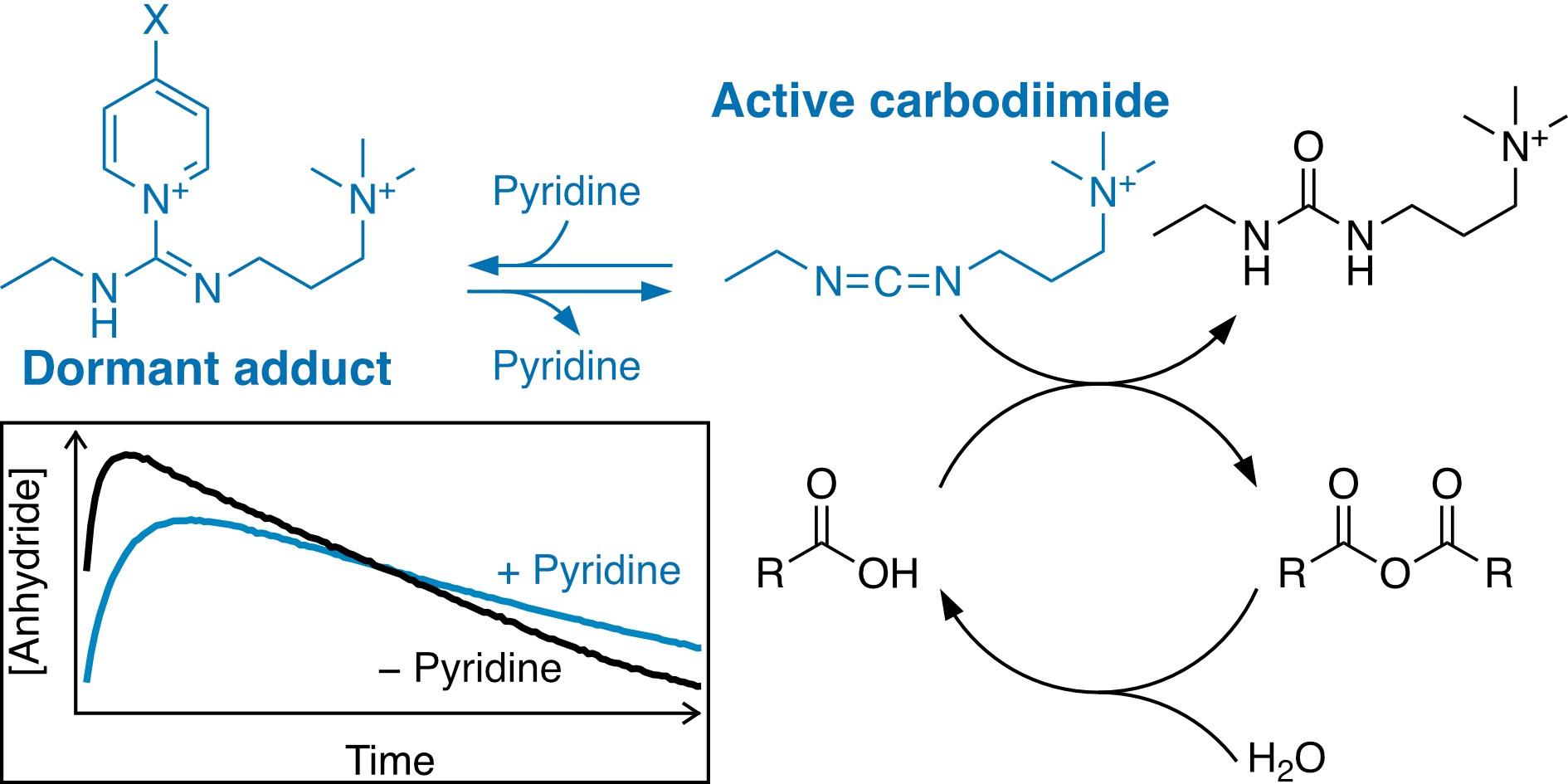
Carbodiimide-driven anhydride formation from carboxylic acids is useful in a variety of non-equilibrium systems. While multiple strategies to control deactivation rates (anhydride hydrolysis) have been reported, control over activation rates (anhydride formation) is currently limited. We show that pyridines reversibly form adducts with 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide methiodide in water. These adducts are unreactive with carboxylic acids and thus reduce the anhydride formation rate while prolonging carbodiimide lifetime. The best results are obtained with 4-methoxypyridine. This strategy can be used to control the formation of transient polymer network hydrogels, in one example increasing the time to reach peak modulus by 86% and the lifetime by 43%.
