Lasith S. Kariyawasam and C. Scott Hartley*
J. Am. Chem. Soc. 2017, 139, 11949–11955
[Published version]
Abstract
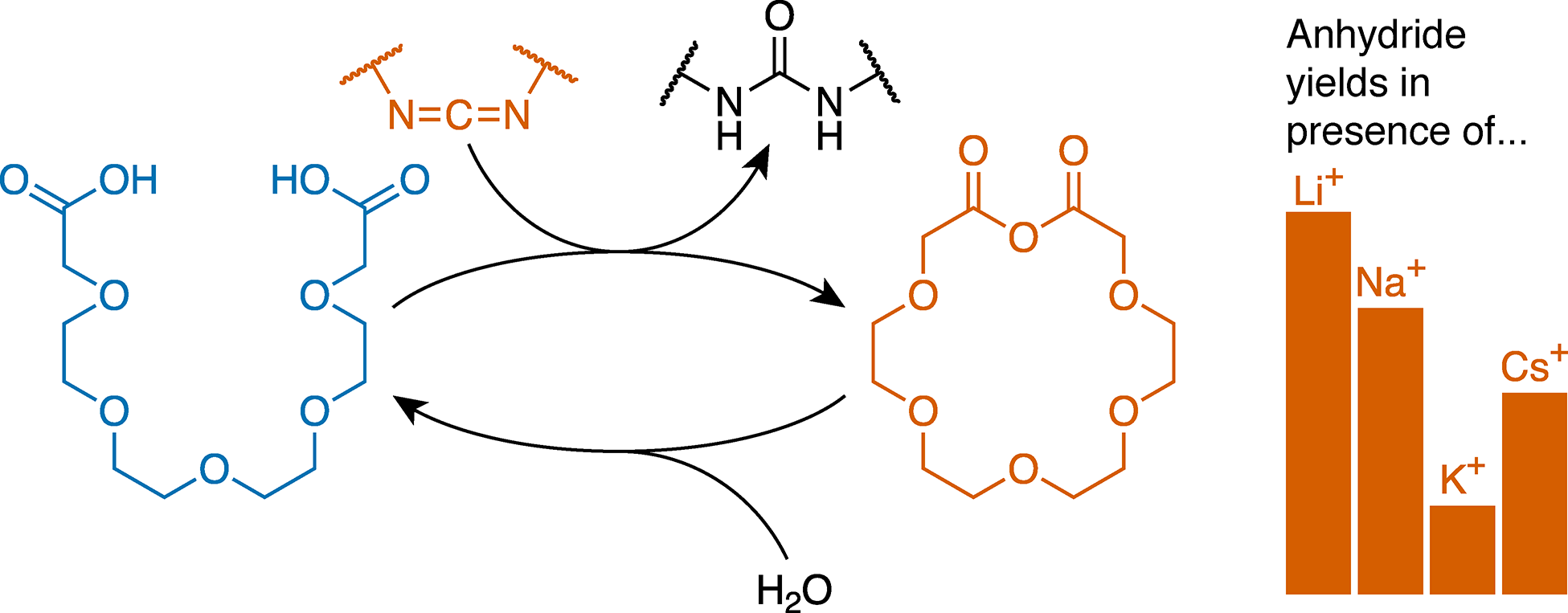
Biochemical systems make extensive use of chemically fueled processes (e.g., using ATP), but analogous abiotic systems remain rare. A key challenge is the identification of transformations that can be adapted to a range of applications and make use of readily available chemical fuels. In this context, the generation of transient covalent bonds is a fundamental tool for nonequilibrium systems chemistry. Here, we show that carbodiimides constitute a simple class of chemical fuels for dissipative assembly, taking advantage of their known reactivity to produce (hydrolytically unstable) anhydrides from carboxylic acids in water. Both aliphatic and aromatic anhydrides are formed on convenient time scales using the common, commercially available peptide coupling agent 1-ethyl-3-(3-(dimethylamino)propyl)carbodiimide (EDC). An important feature of this reaction is that no part of the carbodiimide is incorporated into the transient species; that is, the fuel is decoupled from the structure—and thus function—of the assembled state. We show that intramolecular anhydride formation of oligo(ethylene glycol) diacids gives macrocycles analogous to crown ethers, representing minimal examples of out-of-equilibrium supramolecular hosts. The kinetics and yields of macrocycle formation respond to cation guests, with the presence of matched cations decreasing their overall production.
