Sumalatha Peddi, Juliana M. Livieri, Gopi Nath Vemuri, and C. Scott Hartley*
J. Org. Chem. 2023, 88, 788–795
[Published version | NSF-PAR | Preprint | Raw data]
Abstract
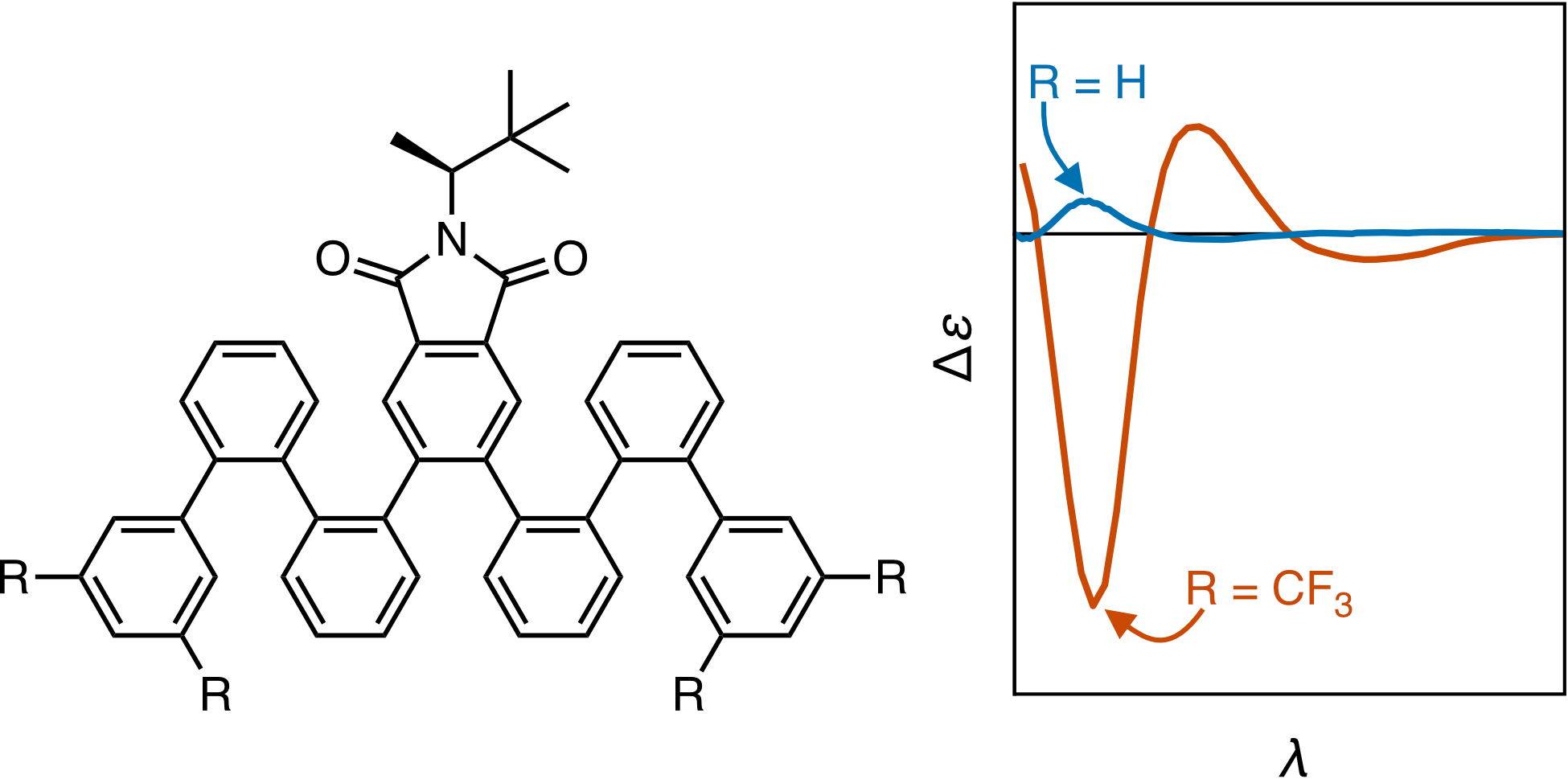
Work on foldamers, non-biological oligomers that mimic the hierarchical structure of biomacromolecules, continues to yield new architectures of ever increasing complexity. o-Phenylenes, a class of helical aromatic foldamers, are well-suited to this area because of their structural simplicity and the straightforward characterization of their folding in solution. However, control of structure requires, by definition, control over folding handedness. Control over o-phenylene twist sense is currently lacking. While chiral induction from groups at o-phenylene termini has been demonstrated, it would be useful to instead direct twisting from internal positions in order to leave the ends free. Here, we explore chiral induction in a series of o-phenylenes with chiral imides at their centers. Conformational behavior has been studied by NMR and CD spectroscopies and DFT calculations. Chiral induction in otherwise unfunctionalized o-phenylenes is generally poor. However, strategic functionalization of the helix surface with trifluoromethyl and methyl groups allows it to better interact with the imide groups, greatly increasing diastereomeric excesses. The sense of chiral induction is consistent with computational models that suggest that it primarily arises from a steric effect.
