C. Scott Hartley*
Acc. Chem. Res. 2016, 49, 646–654
[Published version]
Abstract
In nature, the folding of oligomers and polymers is used to generate complex three-dimensional structures, yielding macromolecules with diverse functions in catalysis, recognition, transport, and charge- and energy-transfer. Over the past 20–30 years, chemists have sought to replicate this strategy by developing new foldamers: oligomers that fold into well-defined secondary structures in solution. A wide array of abiotic foldamers have been developed, ranging from non-natural peptides to aromatics.
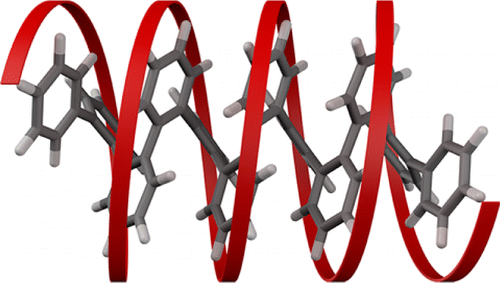
The ortho-phenylenes represent a recent addition to the family of aromatic foldamers. Despite their structural simplicity (chains of benzenes connected at the ortho positions), it was not until 2010 that systematic studies of o-phenylenes showed that they reliably fold into helices in solution (and in the solid state). This conformational behavior is of fundamental interest: o-Arylene and o-heteroarylene structures are found embedded within many other systems, part of an emerging interest in sterically congested polyphenylenes. Further, o-phenylenes are increasingly straightforward to synthesize because of continuing developments in arene–arene coupling, the Asao–Yamamoto benzannulation, and benzyne polymerization. In this Account, we discuss the folding of o-phenylenes with emphasis on features that make them unique among aromatic foldamers. Interconversion between their different backbone conformers is slow on the NMR time scale around room temperature. The 1H NMR spectra of oligomers can therefore be deconvoluted to give sets of chemical shifts for different folding states. The chemical shifts are both highly sensitive to conformation and readily predicted using ab initio methods, affording critical information about the conformational distribution.
The picture that emerges is that o-phenylenes fold into helices with offset stacking between every third repeat unit. In general, misfolding occurs primarily at the oligomer termini (i.e., “frayed ends”). Because of their structural simplicity, the folding can be described by straightforward models. The overall population can be divided into two enantiomeric pools, with racemization and misfolding as two distinct processes. Examination of substituent effects on folding reveals that the determinant of the relative stability of different conformers is (offset) aromatic stacking interactions parallel to the helical axis. That is, the folding of o-phenylenes is analogous to that of α-helices, with aromatic stacking in place of hydrogen bonding. The folding propensity can be tuned using well-known substituent effects on aromatic stacking, with moderate electron-withdrawing substituents giving nearly perfect folding. The combination of a simple folding mechanism and readily characterized conformational populations makes o-phenylenes attractive structural motifs for incorporation into more-complex architectures, an important part of the next phase of foldamer research.
