Nicole M. Dickson-Karn,* Courtney M. Olson, Wade C. W. Leu, and C. Scott Hartley
J. Phys. Org. Chem. 2014, 27, 661–669
[Published version]
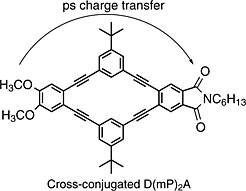
Abstract
An understanding of intramolecular charge transfer in 2-D linearly conjugated and cross-conjugated compounds is necessary for the rational design of molecular electronics, improved solar energy devices, semi-conducting polymers, and materials with nonlinear optical properties. In this work, the femtosecond transient absorption spectra and kinetics of several donor-bridge-acceptor compounds containing cross-conjugated or linearly conjugated bridging groups were obtained. The veratrole group was used as the donor, and the phthalimide group was used as an acceptor. 2-D conjugation was achieved by involving two bridging groups arranged cyclically between the donor and acceptor. The donor and acceptor were bridged by m-phenylene in the cross-conjugated compounds or 2,5-thiophene in the linearly conjugated compounds. We found slower charge separation times and slower charge recombination times in the compounds containing cyclic cross-conjugated bridging groups than in those containing the cyclic linearly conjugated groups in polar solvent. Charge separation rates that were found to be dependent on solvent were observed in the donor-bridge-acceptor compounds.
