Zacharias J. Kinney and C. Scott Hartley*
Org. Lett. 2018, 20, 3327–3331
[Published version | Raw data]
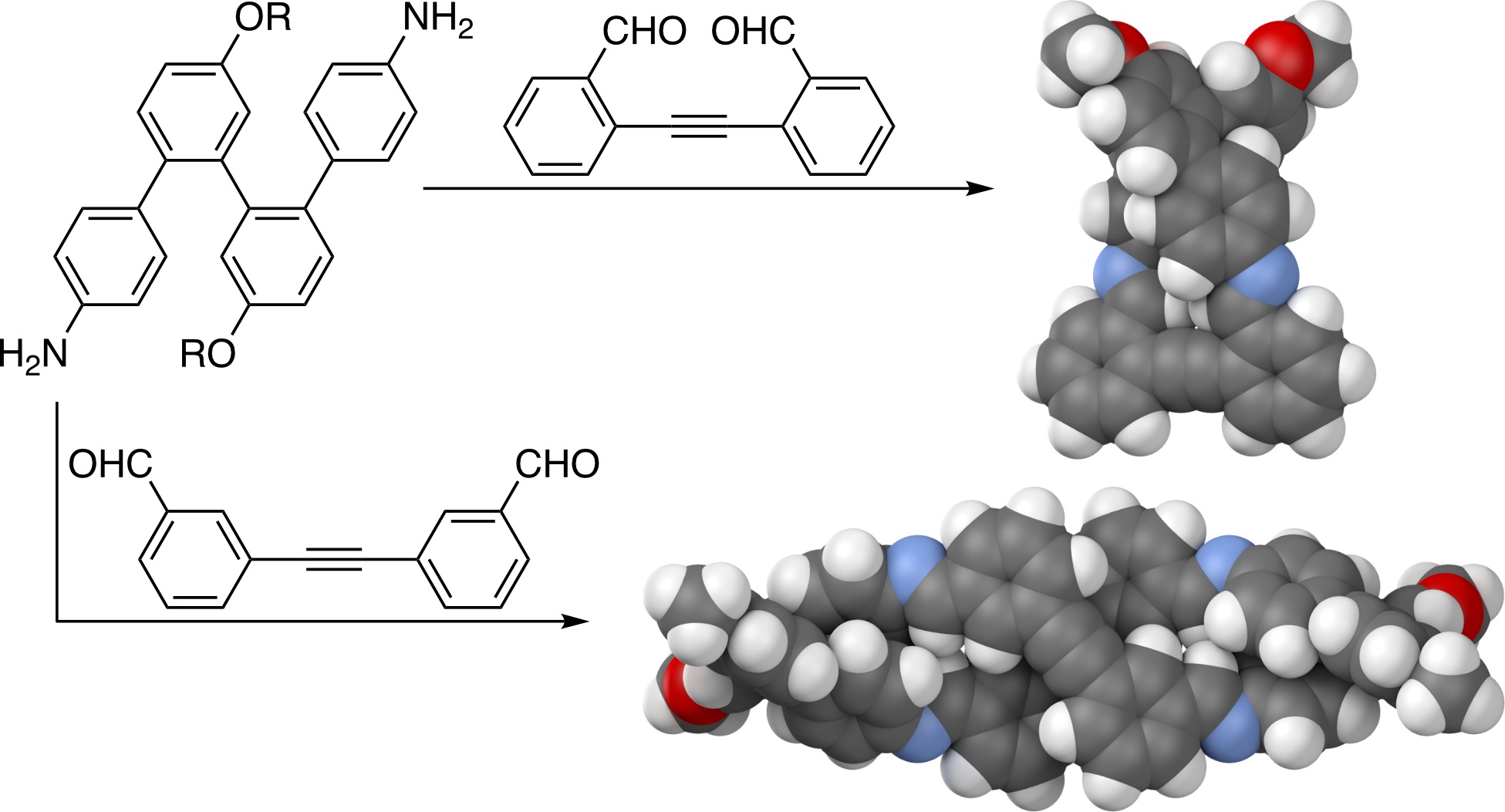
Abstract
o-Phenylene tetramers have been coassembled with linkers into macrocycles through imine condensation. Variation of linker connectivity and length allows both [1 + 1] and [2 + 2] macrocycles to be obtained, complementing (previously reported) [3 + 3] macrocycles. For the [1 + 1] macrocycles, linker length has a clear effect on o-phenylene geometry and macrocycle stability. For the [2 + 2] macrocycles, both homo- and heterochiral configurations are observed, suggesting limited communication of helix handedness in these systems.
