C. Scott Hartley and Jeffrey S. Moore*
J. Am. Chem. Soc. 2007, 129, 11682–11683
[Published version]
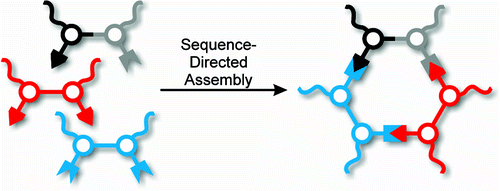
Abstract
Unsymmetrical shape-persistent macrocycles have been prepared from diphenylacetylene monomers using imine formation and metathesis. A sequence-directed approach, in which each monomer is uniquely labeled by its N-donor/C-donor sequence, has been used to control the self-assembly and makes possible an added level of complexity in the final structures. To illustrate the potential of this strategy, a series of macrocycles with different side-chain substitution patterns have been prepared, including monofunctionalized and Janus-type structures. We believe this to be the first example of sequence control of dynamic covalent self-assembly and that it will enable the fully covalent synthesis of more complex nanostructures.
