Wade C. W. Leu, Amanda E. Fritz, Katherine M. Digianantonio, and C. Scott Hartley*
J. Org. Chem. 2012, 77, 2285–2298
[Published version]
Abstract
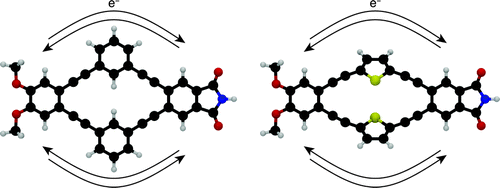
Two-dimensional π-systems are of current interest in the design of functional organic molecules, exhibiting unique behavior for applications in organic electronics, single-molecule devices, and sensing. Here we describe the synthesis and characterization of “push–pull macrocycles”: electron-rich and electron-poor moieties linked by a pair of (matched) conjugated bridges. We have developed a two-component macrocyclization strategy that allows these structures to be synthesized with efficiencies comparable to acyclic donor–bridge–acceptor systems. Compounds with both cross-conjugated (m-phenylene) and linearly conjugated (2,5-thiophene) bridges have been prepared. As expected, the compounds undergo excitation to locally excited states followed by fluorescence from charge-transfer states. The m-phenylene-based systems exhibit slower charge-recombination rates presumably due to reduced electronic coupling through the cross-conjugated bridges. Interestingly, pairing the linearly conjugated 2,5-thiophene bridges also slows charge recombination. DFT calculations of frontier molecular orbitals show that the direct HOMO–LUMO transition is polarized orthogonal to the axis of charge transfer for these symmetrical macrocyclic architectures, reducing the electronic coupling. We believe the push–pull macrocycle design may be useful in engineering functional frontier molecular orbital symmetries.
