Zacharias J. Kinney and C. Scott Hartley*
J. Am. Chem. Soc. 2017, 139, 4821–4827
[Published version]
Abstract
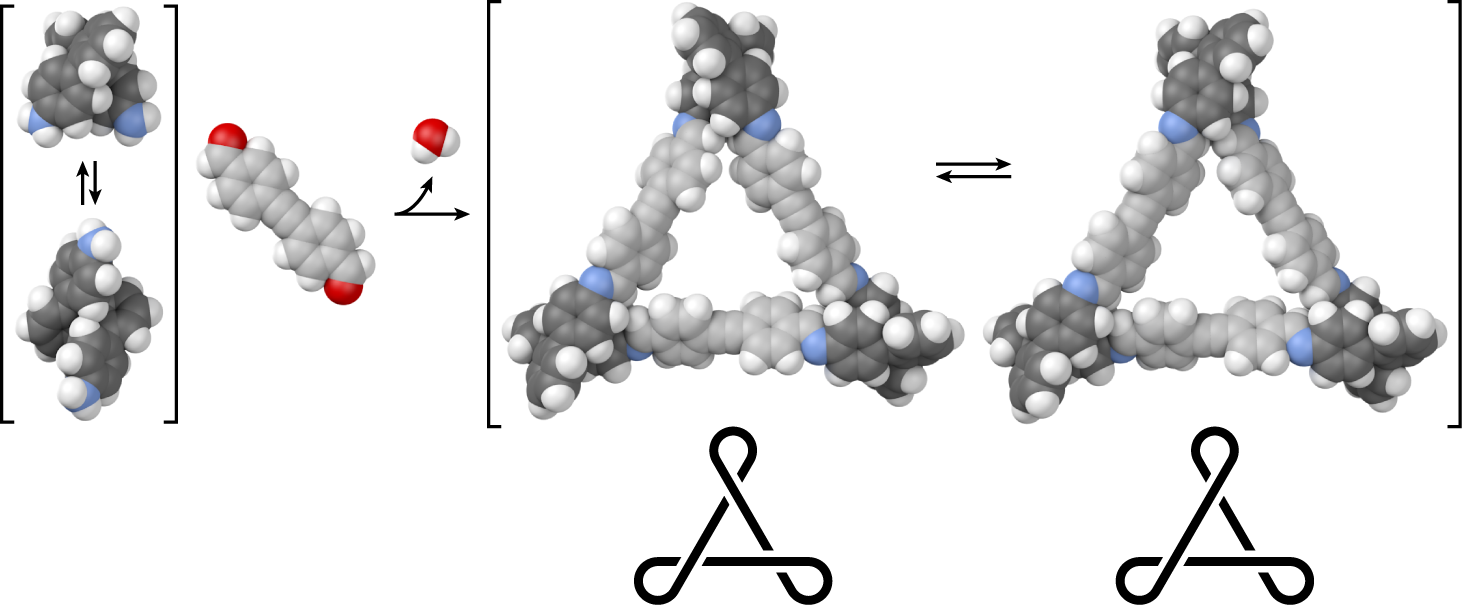
Many foldamers, oligomers that adopt well-defined secondary structures, are now known, including many exhibiting functional behavior. However, examples of foldamer subunits within larger architectures remain rare, despite the importance of higher-order structure in biomacromolecules. Here, we investigate the dynamic covalent assembly of short o-phenylenes, a simple class of aromatic foldamers, into twisted macrocycles. o-Phenylene tetramers have been combined with rod-shaped p-phenylene-, tolane-, and diphenylbutadiyene-based linkers using imine formation. Macrocyclization proceeds efficiently, inducing folding of the o-phenylenes. The resulting [3 + 3] macrocycles (three o-phenylenes and three linkers) are shape-persistent, triangular structures with twisted cores and internal diameters up to approximately 2 nm. The homochiral D3-symmetric and heterochiral C2-symmetric conformers can be distinguished by NMR spectroscopy. Analysis of the conformational distribution for the p-phenylene-linked macrocycle suggests that the o-phenylene units are largely decoupled, with the less-symmetrical configuration therefore entropically favored. Conformational dynamics were assessed by variable-temperature NMR spectroscopy. Confinement within the macrocyclic architecture slows the inversion of the o-phenylene moieties.
