Maia Popova, Stacey Lowery Bretz, and C. Scott Hartley*
J. Chem. Educ. 2016, 93, 1096–1099
[Published version | NSF-PAR]
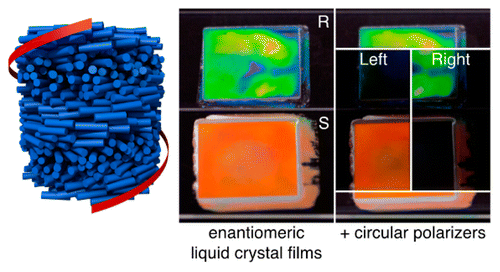
Abstract
Although stereochemistry is an important topic in second-year undergraduate organic chemistry, there are limited options for laboratory activities that allow direct visualization of macroscopic chiral phenomena. A novel, guided-inquiry experiment was developed that allows students to explore chirality in the context of cholesteric liquid crystals. As part of the experiment, which requires no specialized equipment, students visually distinguish two enantiomers. A chiral imine is synthesized in one step from an assigned (but unknown to students) enantiomer of 1-phenylethylamine and then dissolved in a nematic liquid crystal host, inducing a helical structure. The resulting cholesteric liquid crystalline material selectively reflects circularly polarized light with a handedness that depends on the absolute configuration of the starting amine, easily detected using circularly polarizing filters from disposable 3D glasses. Working in teams, students examine the behavior of both dopant enantiomers and the racemic mixture. Analysis of our students’ responses to post-lab questions indicates comprehension of most of the ideas introduced in lab.
